
He also experimented with several metals. He worked with amber, and, in addition, he experimented with rock crystal and various precious and semi-precious gemstones. The English physicist William Gilbert (1544–1603) also studied this attractive force, using various substances. (c) When separated, the amber and cloth now have net charges, but the absolute value of the net positive and negative charges will be equal. (b) When rubbed together, some negative charge is transferred to the amber, leaving the cloth with a net positive charge. Only a tiny fraction of the charges are involved, and only a few of them are shown here. (a) Both the amber and cloth are originally neutral, with equal positive and negative charges. Linear combination can occur between orbitals of two different atoms, creating molecular orbitals, or between two orbitals of the same atom, creating hybrid atomic orbitals.Figure 5.5 When materials are rubbed together, charges can be separated, particularly if one material has a greater affinity for electrons than another. In this process, the total number of orbitals remains constant that is, the number of new orbitals always equals the number of orbitals combined to form them. This combining process is referred to as the linear combination of atomic orbitals. Just as waves can interact to reinforce or diminish themselves, atomic orbitals can combine to form new orbitals. The atomic p orbitals can be oriented along the x, y, and z axes in three‐dimensional space. The shapes of the two most common orbitals found in organic compounds are spherical in atomic s orbitals and hourglass shaped in atomic p orbitals. This region is referred to as an atomic orbital. The first three quantum numbers define the region in space about the nucleus of an atom where there is the highest probability of finding the electron density. The fourth quantum number, the spin quantum number ( m s), describes the direction of spin of the electron. The third quantum number, the magnetic quantum number ( m), describes the orientation of the probability region in space. It corresponds to the orbital designations. The second quantum number, the angular momentum quantum number ( l), tells the shape of the probability region. It corresponds to the orbit, or shell, designation. The first quantum number, the principal quantum number ( n), tells the location of the probability region relative to the nucleus. This equation allowed the calculation of a probability function whose solution generated four quantum numbers. In 1926, Erwin Schrödinger derived a wave equation that incorporated both the particle and wave characteristics of the electron. The point where the wave crosses the original position is called a node, a point of zero amplitude in the wave.
DISCRETE CHARGE OF ELECTRON PLUS
The upward displacement is assigned a plus sign, while the downward one is assigned a negative sign. The string rebounds to its origin and is then displaced in the opposite direction by the same distance. Viewed in slow motion, the plucking of a guitar string first displaces it a certain distance from its original position. A standing wave is basically a stationary, bound vibration-the vibration of a guitar string, for example. In other words, the electron distribution in an atom can be described by the mathematical formulas and physical concepts of a standing wave. Mathematically, this probability distribution is similar to an equation that describes a wave. What can be determined is a region of space around the nucleus where there is a high probability of finding the negative charge of an atom.
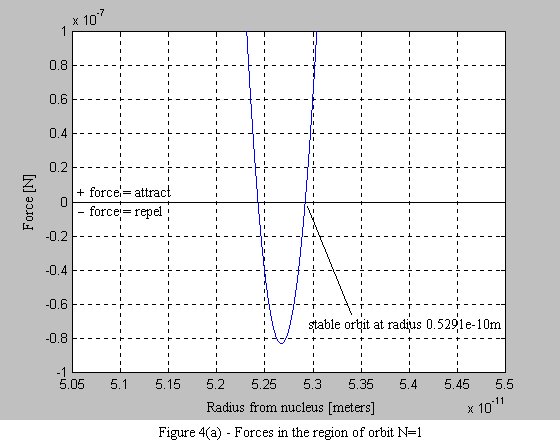
According to a basic principle of quantum mechanics, it is impossible to know simultaneously both the exact position and momentum of an electron this means that the trajectory of the electron cannot be precisely determined. The results of scientific experiments suggest that electrons act more like electromagnetic waves than orbiting particles. The Bohr model of the atom, in which electrons orbit about the nucleus, is a convenient representation. Cahn‐Ingold‐Prelog RS Notational System.
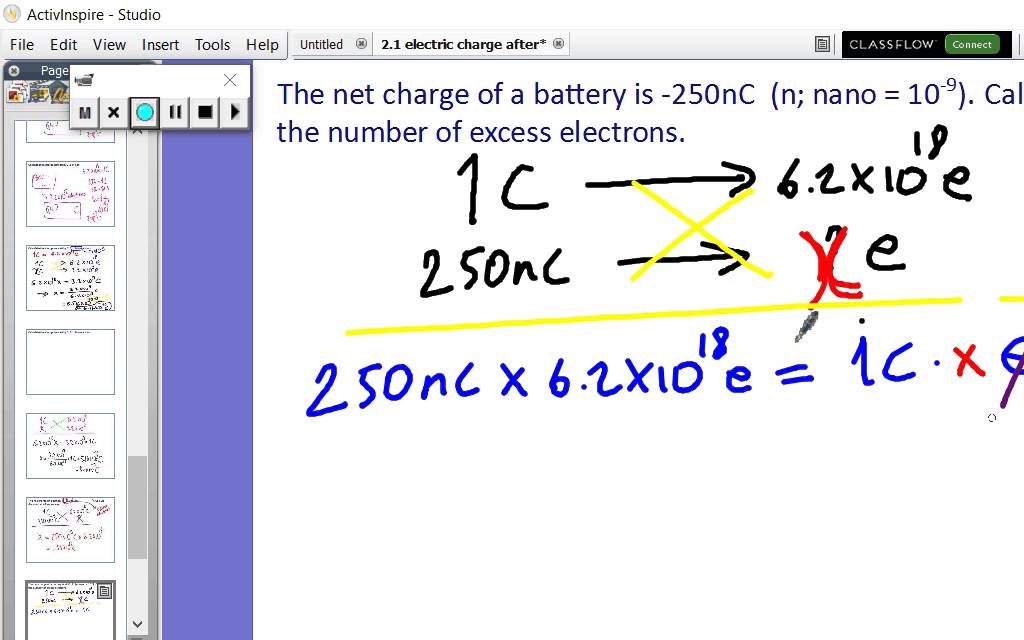

Brønsted‐Lowry Theory of Acids and Bases.


 0 kommentar(er)
0 kommentar(er)
